 打印本文
打印本文  关闭窗口
关闭窗口 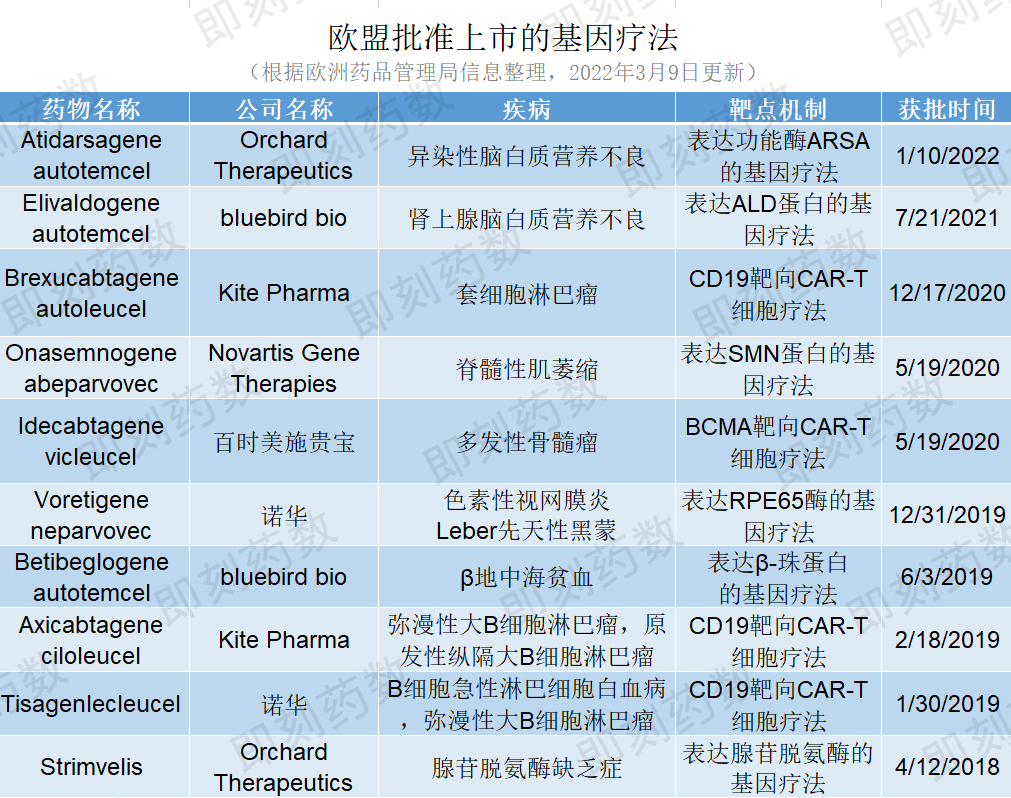
(药明康德内容团队制图,点击可见大图)
值得一提的是,今年有6个基因疗法可能在欧盟取得重大进展。其中,4个基因疗法有望获得欧盟批准,2个基因疗法提交上市授权申请(Marketing Authorisation Application,MAA),详见下表。
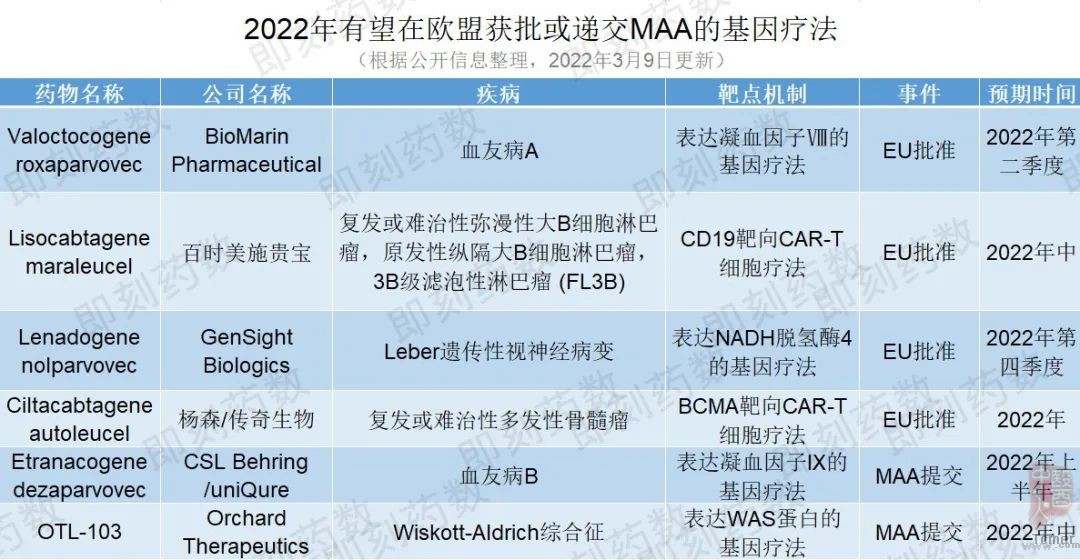
(药明康德内容团队制图,点击可见大图)
2022年在欧盟预期获批的基因疗法
第一个预期获批的基因疗法是BioMarin的valoctocogene roxaparvovec,一种基于AAV5载体的基因疗法,用于治疗严重的血友病A。该公司最近提交了积极的2年疗效数据以支持其MAA申报,预计人用药品委员会(Committee for Medicinal Products for Human Use,CHMP)意见将在2022年第二季度获得。如果获得批准,这可能是潜在的第一个治疗血友病A的基因疗法,根据公司发布信息,这款药物预计将于2022年第二季度获得批准。
另一个有希望获得批准的候选药物是百时美施贵宝的lisocabtagene maraleucel,一种以CD19为靶点的CAR-T细胞疗法,用于二线或多线全身治疗后的成人复发或难治性弥漫性大B细胞淋巴瘤(DLBCL)、原发性纵隔大B细胞淋巴瘤(PMBCL),滤泡性淋巴瘤3B级(FL3B)患者。CHMP在1月给出支持批准的积极意见,预计欧盟将在2022年年中做出批准决定。该疗法已获美国FDA批准。
同样值得关注的是GenSight Biologics的lenadogene nolparvovec,一种编码人类野生型ND4蛋白的AAV2基因疗法,用于治疗Leber遗传性视神经病变,这是一种罕见的线粒体疾病,在青少年时就会发病,导致不可逆转的失明。欧盟目前正在对提交的MAA进行监管审评。GenSight预计该申请将在2022年第四季度获得批准,并计划在2023年第一季度推出此治疗方式。
最后一个潜在的欧盟批准候选药物是杨森和传奇生物的ciltacabtagene autoleucel,一种靶向B细胞成熟抗原(BCMA)的自体CAR-T细胞免疫疗法,用于治疗复发或难治性多发性骨髓瘤。目前公司正在等待对其MAA的监管决定。这种疗法已经成为杨森在美国获批的首个细胞疗法。如果在欧盟获得批准,该疗法可能成为欧盟第二个针对多发性骨髓瘤的BCMA靶向细胞疗法。
2022年在欧盟预期提交MAA的基因疗法
除了潜在的批准外,预计今年还会有两种基因疗法会进行MAA的提交。uniQure和CSL Behring的etranacogene dezaparvovec是一种基于AAV5载体的基因疗法,处于3期临床试验中,用于治疗血友病B,这是一种危及生命的出血性疾病。CHMP已准许使用加速评估程序对这一MAA进行审评,计划于2022年上半年进行。一旦MAA提交完成并被欧盟监管机构接受,加速评估可能会将审查时间从210天缩短到150天。
Orchard Therapeutics的OTL-103是一种离体、自体、造血干细胞(HSC)基因疗法,用于治疗Wiskott-Aldrich综合征,这是一种罕见的遗传性免疫缺陷疾病,目前已知的治疗方法是干细胞移植。在与EMA进行有效的监管互动后,该公司计划在2022年年中提交MAA。
参考资料:
[1] BioMarin Announces Fourth Quarterand Full Year 2021 Financial Results and Corporate Updates. Retrieved March 8, 2022 from, https://investors.biomarin.com/2022-02-23-BioMarin-Announces-Fourth-Quarter-and-Full-Year-2021-Financial-Results-and-Corporate-Updates
[2] BioMarin Provides Updates onProgress in Gene Therapy Programs. Retrieved March 8, 2022 from, https://investors.biomarin.com/2022-02-17-BioMarin-Provides-Updates-on-Progress-in-Gene-Therapy-Programs
[3] Bristol Myers Squibb ReceivesPositive CHMP Opinion for CAR T Cell Therapy Breyanzi (lisocabtagenemaraleucel) for Relapsed or Refractory DLBCL, PMBCL and FL3B. Retrieved March 8, 2022 from, https://news.bms.com/news/corporate-financial/2022/Bristol-Myers-Squibb-Receives-Positive-CHMP-Opinion-for-CAR-T-Cell-Therapy-Breyanzi-lisocabtagenemaraleucel-for-Relapsed-or-Refractory-DLBCL-PMBCL-and-FL3B/default.aspx
[4] Corporate Presentation of GenSight Biologics. Retrieved March 8, 2022 from, https://www.gensight-biologics.com/wp-content/uploads/2022/02/GenSight-Biologics-Corporate-presentation-February-2022-v25022022.pdf
[5] U.S. FDA Approves CARVYKTI™(ciltacabtagene autoleucel), Janssen’s First Cell Therapy, a BCMA-DirectedCAR-T Immunotherapy for the Treatment of Patients with Relapsed or RefractoryMultiple Myeloma. Retrieved March 8, 2022 from, https://www.janssen.com/us-fda-approves-carvykti-ciltacabtagene-autoleucel-janssens-first-cell-therapy-bcma-directed-car-t
[6] GenScript Biotech Reports FirstHalf 2021 Results Posts explosive growth in GCT CDMO business and strategic GCTinvestments. Retrieved March 8, 2022 from, https://www.genscript.com/genscript-biotech-2021-interim-results-presentation.html?page_no=1&position_no=2&sensors=search
[7] First cell-based gene therapy totreat adult patients with multiple myeloma. Retrieved March 8, 2022 from, https://www.ema.europa.eu/en/news/first-cell-based-gene-therapy-treat-adult-patients-multiple-myeloma
[8] CSL Behring Receives AcceleratedCHMP Assessment for Etranacogene Dezaparvovec for European Patients Living withHemophilia B. Retrieved March 8, 2022 from, https://www.cslbehring.com/newsroom/2021/chmp-accelerates-etranadez-application
[9] uniQure and CSL Behring AnnouncePrimary Endpoint Achieved in HOPE-B Pivotal Trial of Etranacogene DezaparvovecGene Therapy in Patients with Hemophilia B. Retrieved March 8, 2022 from, https://www.cslbehring.com/newsroom/2021/hope-b-gene-therapy-for-hemophilia-b-topline-results
[10] Orchard Therapeutics AnnouncesRecent Commercial and Regulatory Progress for Late-stage HSC Gene TherapyPrograms and Outlines Key 2022 Milestones. Retrieved March 8, 2022 from, https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-recent-commercial-and-regulatory
[11] Pipeline of Orchard Therapeutics.Retrieved March 8, 2022 from, https://www.orchard-tx.com/approach/pipeline/
[12] Wiskott-Aldrich Syndrome. Retrieved March 8, 2022 from, https://www.childrenshospital.org/conditions-and-treatments/conditions/w/wiskott-aldrich-syndrome
 打印本文
打印本文  关闭窗口
关闭窗口