硫酸羟氯喹的临床药理学研究进展
7 讨论
7.1 硫酸羟氯喹与磷酸氯喹的临床药理学特征比较
硫酸羟氯喹与磷酸氯喹同属于4-氨基喹啉类抗疟药,由两个芳香环构成。二者的区别在于氯喹中的一个乙基在羟氯喹中被一个羟乙基所代替,增加了水溶性,促进了药物在人体的吸收,分布更广,不良反应发生率更低。根据上文综述,两个药物的主要临床药理学特征比较见Tab.2。
Tab.2 Comparison of clinical pharmacologic characteristics between chloroquine and hydroxychloroquine 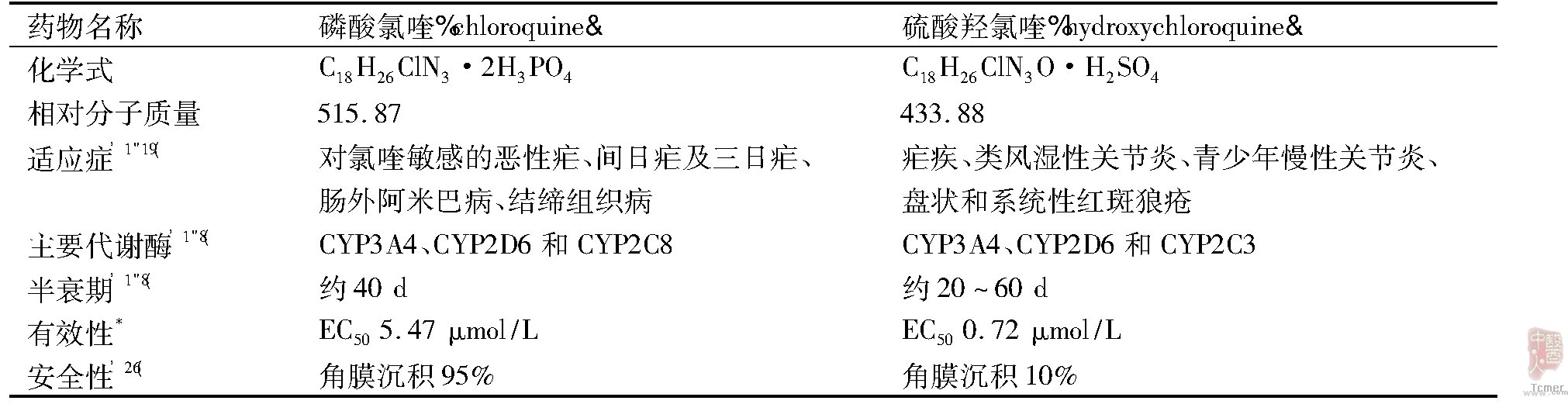
*平行体外抑制新冠病毒活性(48 h孵育结果,结果待发表)。
7.2 已上市药物增加适应症临床治疗剂量的选择建议
对于某些特殊疾病和医疗紧急情况(比如突发恐怖事件或重大疫情),许多人体药物临床试验无法正常或者及时开展,研究者需依赖新药的动物药代/药效(PK/PD)学数据外推新药在人体的PK/PD等特征,并基于外推的人体PK/PD特征而支持上市,从而治疗患者。这种被FDA定义为动物法则(animal rule)[33]的申报上市路径已经在炭疽治疗中被接受。
鉴于目前紧张抗疫形式,我国并无时间按照常规新药临床开发中要求的临床早期开发过程(即从临床前到临床I期,从临床I期到临床II期试验)对已上市药物更改治疗适应症进行剂量确定。此时,本文作者认为可以借鉴美国的动物法则,充分利用临床药理学与定量药理学原理与技术,整合已有细胞水平和动物水平的数据以及临床安全性数据建立剂量-药物浓度-效应(包括有效性和安全性)定量关系,以此定量关系快速建立新药从细胞/动物水平数据,跨过一些常规新药研发的剂量优化步骤(动物毒理药代,I期安全性和药动学试验),直接到患者水平剂量验证的转化桥梁,以支持新药尽快、科学地被应用于临床患者治疗中。
基于此原理,本研究室正在利用定量药理学技术建立生理药动学模型(定量药理学的重要研究技术之一),结合已发表的氯喹/羟氯喹动物与已知人体药代动力学特征对该生理药动学模型进行验证,并预测两者肺组织药物浓度。然后依据预测的氯喹肺组织药物浓度和血液药物浓度针对临床需求进行给药方案优化,临床治疗结果可以及时反馈到模型中进行验证和必要的改善,并通过模型指导是否需要对给药方案进行调整。
7.3 硫酸羟氯喹治疗COVID-19的给药建议
新适应症剂量的快速确定可参考美国FDA的动物法则,应用生理药动学模型。由于羟氯喹的半衰期较长,基于生理药动学模型预测药物在肺组织容易高度蓄积,并且代谢消除缓慢。参考羟氯喹的抗疟治疗方案和类风湿关节炎治疗方案,首日给予一定的负荷剂量可使药物快速达到有效暴露水平,同时降低维持剂量避免药物在组织的长期高度蓄积,并根据疾病进程调整给药周期,以合理制定COVID-19的治疗方案。基于上述药物特性,建议用药3~7 d,并持续观察至少10~30 d,在观察期严禁服用莫西沙星等喹诺酮类药物和大环内酯类抗生素。同时,应避免空腹服用药物,建议随餐或牛奶服用,提高患者的耐受性。
参考文献
[1] 么雪婷,崔诚,刘东阳,等.氯喹抗新型冠状病毒肺炎临床药理学综述[J].临床药物治疗杂志,2020,18(2) (已接收).
[2] 夏瑾瑜.临床试验方案:氯喹和洛匹那韦/利托那韦片治疗轻/普通型新型冠状病毒(2019-nCoV)感染者的疗效研究:一项前瞻性、开放性、多中心随机对照临床研究[EB/OL].(2020-02-13).http://www.chictr.org.cn/showproj.aspx?proj=49263.
[3] 国家卫生健康委员会.关于印发新型冠状病毒肺炎诊疗方案(试行第六版)的通知[EB/OL].(2020-02-19).http://www.nhc.gov.cn/yzygj/s7653p/202002/ 8334a8326dd94d329df351d7da8aefc2.shtml?from=timeline.
[4] 广东省科技厅及广东省卫生健康委磷酸氯喹治疗新冠状病毒肺炎多中心协作组.磷酸氯喹治疗新型冠状病毒肺炎的专家共识[J].中华结核和呼吸杂志,2020,43(00):E019-E019.
[5] Plantone D,Koudriavtseva T.Current and future use of chloroquine and hydroxychloroquine in infectious,immune,neoplastic,and neurological diseases:a mini-review[J].Clin Drug Investig,2018,38(8):653-671.
[6] Kersh GJ.Antimicrobial therapies for Q fever[J].Expert Rev Anti Infect Ther,2013,11(11):1207-1214.
[7] Keshavarzi F.Fungistatic effect of hydroxychloroquine,lessons from a case[J].Med Mycol Case Rep,2016,13:17-18.
[8] Ponticelli C,Moroni G.Hydroxychloroquine in systemic lupus erythematosus (SLE) [J].Expert Opin Drug Saf,2017,16(3):411-419.
[9] Savarino A,Di TL,Donatelli I,et al A.New insights into the antiviral effects of chloroquine [J].Lancet Infect Dis,2006,6:67-69.
[10] Wang LF,Lin YS,Huang NC,et al.Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery[J].J Interferon Cytokine Res,2015,35(3):143-156.
[11] Cao B,Parnell LA,Diamond MS,et al.Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice[J].J Exp Med,2017,214(8):jem.20170957.
[12] Paton NI,Goodall RL,Dunn DT,et al.Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy:a randomized controlled trial [J].JAMA,2012,308(4):353-361.
[13] Piconi S,Parisotto S,Rizzardini G,et al.Hydroxychloroquine drastically reduces immune activation in HIV-infected,antiretroviral therapy-treated immunologic nonresponders[J].Blood,2011,118(12):3263-3272.
[14] Wang M,Cao R,Zhang L,et al.Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus(2019-nCoV)in vitro [J].Cell Res,2020,doi:10.1038/s41422-020-0282-0.
[15] Wolstencroft PW,Casciola-Rosen L,Fiorentino DF.Association between autoantibody phenotype and cutaneous adverse reactions to hydroxychloroquine in dermatomyositis [J].JAMA Dermatol,2018,154(10):1199-1203.
[16] Casian A,Sangle SR,D'cruz DP.New use for an old treatment:Hydroxychloroquine as a potential treatment for systemic vasculitis[J].Autoimmun Rev,2018,17(7):660-664.
[17] Pareek A,Chandurkar N,Thomas N,et al.Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus:a double blind,randomized comparison with pioglitazone [J].Curr Med Res Opin,2014,30(7):1257-1266.
[18] Manic G,Obrist F,Kroemer G,et al.Chloroquine and hydroxychloroquine for cancer therapy[J].Mol Cell Oncol,2014,1(1):e29911.
[19] FDA.FDA label [EB/OL].[2020-02-26].https://www.accessdata.fda.gov/ drugsatfda_docs/label/2019/009768Orig1s051lbl.pdf.
[20] 白云静,姜德训,申洪波,等.硫酸羟氯喹的不良反应临床调查分析[J].北京医学,2011,33(7):575-577.
[21] Costedoat-Chalumeau N,Dunogué B,Leroux G,et al.A critical review of the effects of hydroxychloroquine and chloroquine on the eye [J].Clin Rev Allergy Immunol,2015,49:317-326.
[22] Mavrikakis M,Papazoglou S,Sfikakis PP,et al.Retinal toxicity in long term hydroxychloroquine treatment [J].Ann Rheumatic Dis,1996,55(3):187-189.
[23] Martin R,Howard NB,Nathan JZ.Studies on the pharmacology of chloroquine:Recommendations for the treatment of chloroquine retinopathy [J].Arch Ophthalmol,1963,70:474-481.
[24] Mcchesney EW.Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate[J].Am J Med,1983,75(1):11-18.
[25] Bernstein HN ,Zvaifler NJ ,Rubin M,et al.Ocular deposition of chloroquine[J].Invest Ophthalmol,1963,2(4):384-392.
[26] Furst DE.Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases[J].Lupus,1996,5 Suppl 1:S11-S15.
[27] Tett SE.Clinical pharmacokinetics of slow-acting antirheumatic drugs[J].Clin Pharmacokinet,1993,25(5):392-407.
[28] Fan HW,Ma ZX,Chen J,et al.Pharmacokinetics and Bioequivalence Study of Hydroxychloroquine Sulfate Tablets in Chinese Healthy Volunteers by LC-MS/MS[J].Rheumatol Ther,2015,2(2):183-195.
[29] Browning DJ.Pharmacology of chloroquine and hydroxychloroquine,hydroxychloroquine and chloroquine retinopathy[M].New York,NY:Springer New York,2014:35-63.
[30] Munster T,Gibbs JP,Shen D,et al.Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis[J].Arthritis Rheum,2002,46(6):1460-1469.
[31] Leden I.Digoxin-hydroxychloroquine interaction [J]?Acta Med Scand,1982,211(5):411-412.
[32] Samuels ER,Sevrioukova I.Inhibition of human CYP3A4 by rationally designed ritonavir-like compounds:impact and interplay of the side group functionalities[J].Mol Pharm,2017,15(1):279-288.
[33] Bergman KL.The animal rule:The role of clinical pharmacology in determining an effective dose in humans [J].Clin Pharmacol Ther,2015,98(4):365-368.